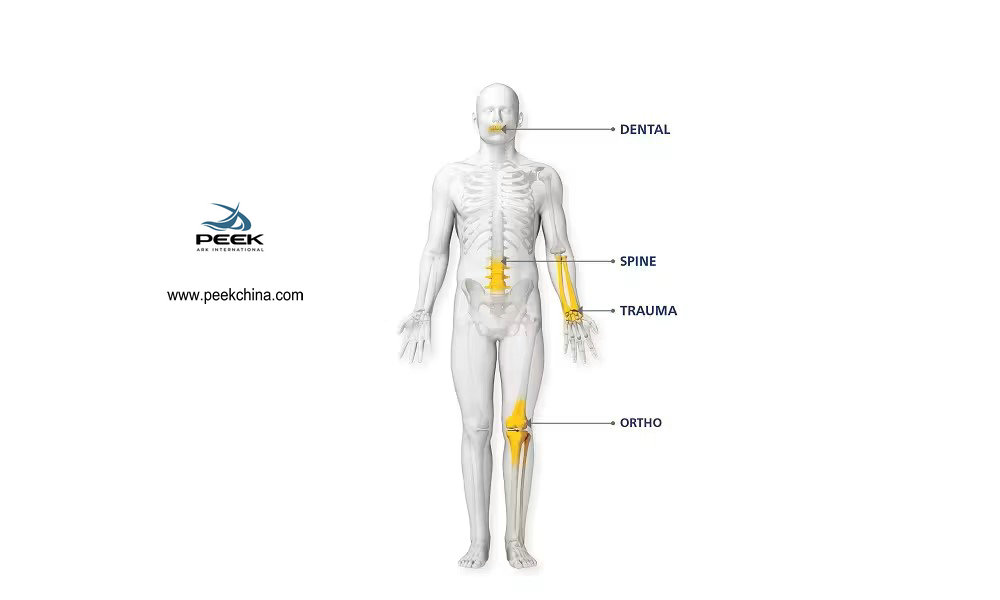
Introduction
With the advancement of medical technology, the requirements for medical device materials are becoming increasingly stringent. PEEK material, due to its unique physical and chemical properties, has become an important new material in the medical device field. This article will explore the current application status of PEEK in the medical device industry and its potential advantages.
Properties of PEEK Material
PEEK is a semi-crystalline high polymer with the following characteristics:
Elastic modulus similar to human bone: PEEK's elastic modulus is very close to that of bones, avoiding the stress shielding issue present in metal implants.
Heat resistance: PEEK has a melting point of up to 343°C and can be used in hot water or steam under pressure at 300°C. PEEK can withstand up to 3,000 cycles of high-pressure sterilization at 134°C, making it suitable for the production of surgical and dental equipment that require high sterility and repeated use.
Chemical resistance: PEEK has excellent resistance to most chemicals and is not easily corroded.
Biocompatibility: PEEK does not cause adverse reactions in the human body, making it suitable as an implant material.
X-ray transparency: PEEK has good X-ray permeability, does not produce metal artifacts, and facilitates postoperative observation.
Applications of PEEK in Medical Devices
1, Orthopedic Implants
PEEK's high mechanical strength, elastic modulus close to human bone, and biocompatibility make it an ideal material for orthopedic implants. For example, PEEK can be used to manufacture spinal fusion devices, joint replacement parts, etc., which can provide good support and stability within the body.
2, Dental Applications
In the dental field, PEEK is used to manufacture dental crowns, bridges, and implants. PEEK's aesthetics and biocompatibility make it a preferred material for tooth restoration.
3, Cardiovascular Devices
PEEK's heat resistance and chemical resistance make it suitable for the manufacture of cardiovascular devices, such as heart valves and vascular stents, which need to function stably within the body for long periods.
4, Surgical Tools
PEEK's durability and high-temperature resistance make it an ideal choice for manufacturing surgical tools and instruments, such as surgical knife handles and endoscopes.
5, Drug Delivery Systems
PEEK's chemical stability and biocompatibility make it suitable for drug delivery systems, such as implantable drug pumps and controlled release systems.
Challenges and Prospects
Despite the broad application prospects of PEEK in the medical device industry, there are some challenges, such as high costs and processing difficulties. However, the emergence of PEEK 3D printing technology has improved this situation. 3D printing technology results in less material waste, significantly reducing costs. The customizable nature of 3D printing further broadens the application scope of PEEK in medical settings. With technological advancements and cost reductions, the application of PEEK is expected to become more widespread.
PEEK, with its excellent performance, plays an increasingly important role in the medical device industry. As research into PEEK materials deepens, its applications in the medical field will further expand, providing patients with more treatment options and better therapeutic outcomes.
RFQ
What is the medical abbreviation PEEK?
Polyetheretherketone (PEEK) is a polyaromatic semi-crystalline thermoplastic polymer with mechanical properties favorable for bio-medical applications.
What is a PEEK in medical terms?
PEEK's radiolucency allows for extremely accurate image reading. Another PEEK property that is of particular interest in orthopedics is its lightweight nature. PEEK is much lighter than metal, which allows patients to manipulate joints and limbs with less effort than would be necessary with heavier metals.
What is PEEK in orthopedics?
Polyetheretherketone (PEEK) pronounced (poly-ether-ether-ketone) is an exceptionally strong thermoplastic polymer. Implantable-grade PEEK is an advanced thermoplastic material that is extremely well suited for both short- and long-term implantation. ARKPEEK-MED® and ARK-BioPEEK® have been cleared by the FDA and are CE marked.
What is a PEEK device?
PEEK spinal cages, also termed interbody fusion cages, are used in spinal fusion procedures to replace a damaged spinal disc and provide an ideal environment for two vertebrae to fuse together.
Can PEEK implants be removed?
Due to limited knowledge, PEEK implants should be used with caution and, when removal is needed, the shape and structure of the implant should be taken into consideration when planning the surgical approach.
What are the medical uses of PEEK?
Here are some ways Peek plastics are used in medical devices. Peek is commonly used in orthopedic implants, such as spinal fusion cages, joint replacements, and trauma fixation plates. Its mechanical properties closely resemble those of bone, making it an ideal material for load-bearing applications.
What is PEEK in neurosurgery?
ARK-BioPEEK® Customized Implants are designed individually for each patient to replace bony voids in the cranial and craniofacial skeleton. PEEK implants may provide a strong, rigid reconstruction along with excellent anatomical fit and contour.
What is PEEK in biomedical?
Poly (ether-ether-ketone) (PEEK) is a biocompatible, high-performance polymer and one of the most prominent candidates to be used in manufacturing bone implants due to its similarity to the mechanical properties of bone.
How long does a PEEK implant last?
The results usually last for about 10 years.
Is PEEK hazardous?
PEEK plastic undergoes strict quality control during the production process to ensure that its chemical composition is stable and no harmful substances are released. In addition, PEEK plastic does not release harmful gases at high temperatures, so it does not pose a threat to human health.
Is PEEK FDA approved?
Polyetheretherketone (PEEK) is a thermoplastic polymer approved by the United States (US) Food and Drug Administration (FDA) as a proper material for bone implant production [1]. Polyetheretherketone (PEEK) is one of the most important bone implant material candidates due to the similar mechanical properties to bone.
What is the difference between titanium and PEEK?
Compared to titanium, PEEK has a lower modulus of elasticity, minimizing the stress-shielding effect and providing a more natural load-sharing environment. PEEK anchors also possess radiolucency, which allows better visualization of tissues during post-operative imaging.
What is the infection rate of PEEK implants?
Implant infection was seen in 6% of PEEK cases, which was towards the lower or midpoint of the ranges cited by the authors in the literature (0-25.9% for bone and 0-11% for titanium mesh).
Is PEEK implant safe for MRI?
While acrylic-PEEK implants might offer strength and to a certain extent MRI compatibility (however see Fig. 7, Fig. 8), these implants fall behind in terms of biocompatibility and durability since the acrylic around the implant can introduce complications at the skin margin.
What are the disadvantages of PEEK implants?
Although PEEK has been widely used as the replacements of metallic and ceramic implants in medical field, it is biologically inertia, which reduces the fusion with host bone tissue after implantation. In addition, the high friction coefficient and hydrophobicity of PEEK also seriously hinder its applications.