In the past few decades, the application of plastics in the medical field has become increasingly widespread. Since its first clinical application in 1999, polyetheretherketone (PEEK) has stood out among numerous medical materials, garnering recognition from countless medical device manufacturers and surgeons, and becoming a heavyweight star in the medical materials industry.

PEEK's Medical Performance:
1, Excellent Biocompatibility: PEEK meets the requirements of ISO 10993-1, demonstrating excellent biocompatibility without any adverse effects.
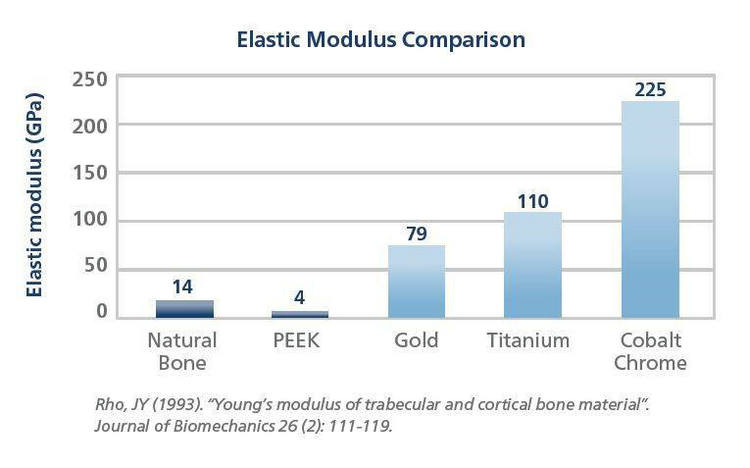
2, Similar Elastic Modulus to Human Bones: PEEK's low elastic modulus, similar to that of bones, effectively mitigates stress shielding effects, maintaining the strength of surrounding bones.
3, X-ray Transparency: PEEK allows X-rays to pass through, providing good postoperative visibility for clear lesion visualization.
4, Corrosion Resistance: PEEK is insoluble in any solvent, strong acid, or strong alkali, except concentrated sulfuric acid, exhibiting high chemical stability.
5, Outstanding Sterilization Performance: PEEK can withstand repeated high-temperature sterilization in pressurized hot water or steam at 300°C, making it suitable for high sterilization requirements in surgical or dental equipment that needs to be reused.
Applications of PEEK in the Medical Field:
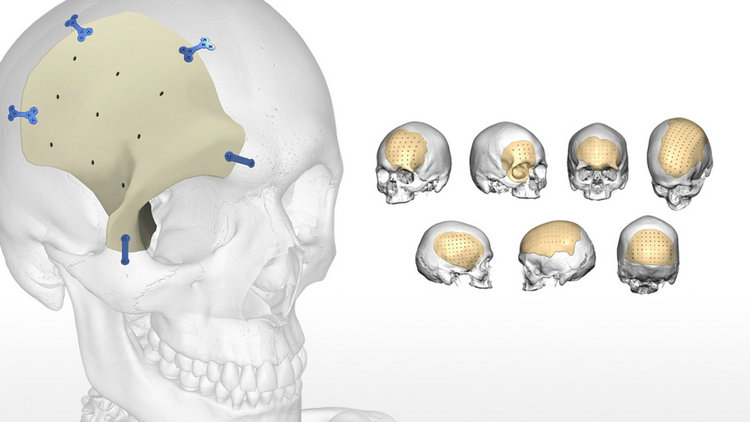
1, Implantable: PEEK implants meet ASTM F2026 requirements and can be used in various clinical applications involving contact with blood, bones, and tissues for over 30 days, such as spinal fusion devices, trauma fracture fixation, orthopedics, craniofacial surgery, oral restoration, as well as valve stents, gastrointestinal stents, implantable fluid pumps, sublingual nerve stimulation systems, etc.
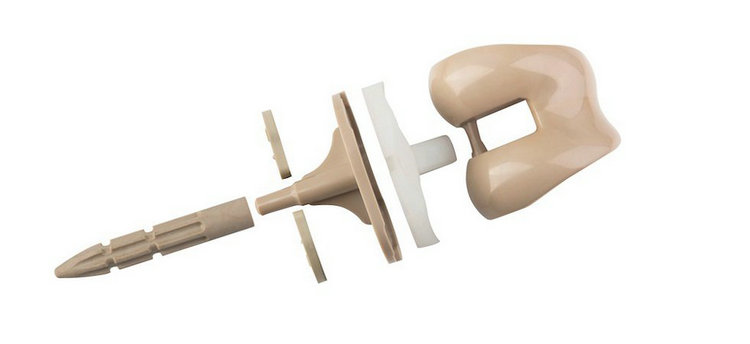
2, Non-implantable: PEEK's excellent chemical stability, strength, stiffness, and toughness, coupled with its ability to withstand repeated high-temperature sterilization, make it suitable for medical device applications such as drug delivery, dental abutments, catheters, blood management, and surgical instruments.
PEEK Market in the Medical Industry:
With an aging population and increased demand for healthcare, the acceptance of high-performance PEEK in the medical field is expected to grow rapidly, leading to broader applications and market penetration.
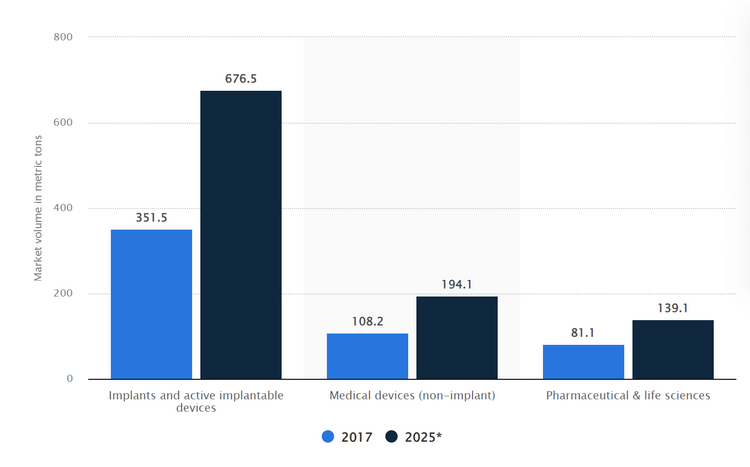
In developed medical technology regions like North America, according to research firm Statista, the medical PEEK market was approximately $176.6 million in 2017 and is projected to reach $335.8 million by 2025, representing a 90% growth.
In China, according to data from Southern Research, in the field of cranioplasty products, from 2020 to 2023, the compound growth rate of the PEEK cranioplasty product market exceeded 80%, showing a trend of replacing titanium materials.
From the application perspective, as a leader in the domestic PEEK cranioplasty field, Kangtuo Medical's semi-annual report shows a rapid increase in PEEK penetration, with its PEEK material neurosurgical products generating revenue of 54.5341 million yuan, a 60.00% increase over the same period last year.
From the material perspective, domestic and foreign PEEK material companies have been dynamic recently:
Victrex signed an agreement to expand PEEK capacity in Shanghai and increase research and development and production lines.
ZYPEEK announced the establishment of a wholly-owned subsidiary, Jilin Hehe Medical Technology Co., Ltd., to expand its research and development scale of medical-grade polyetheretherketone materials and related products.
Solvay increased investment in Shenzhen Collaborative Innovation High-Tech Development Co., Ltd., to deploy 3D printing technology and layout in high-end applications such as implantable medical devices.
Currently, well-knew medical-grade PEEK material manufacturers include Victrex (Invibio), Solvay, Wynn's, Ensinger, ARKPEEK, ZYPEEK, etc.